Which of the Following Describe Chemical Not Physical Changes
Examples of chemical changes from the given list of changes are. Liquid water freezes and turns to ice b.

Physical Changes Chemical Properties And Chemical Changes Quiz Quizizz
If the answer is no its a chemical change.
. Select the correct answer below. Burning things like wood or bread changes their color and odor. Physical change is a temporary change.
Boiling point is known as a physical property because in boiling point youre not changing the composition of matter. When sugar in a beaker is heated over a low flame the solid crystals disappear and a thick yellow-brown liquid forms. A flashlight beam slowly gets dimmer and finally goes our due to a dying battery.
Chemical changes cannot be caused by oxygen Advertisement Answer 48 5 67. Chemical changes involve the formation of a new substance. Dissolving sugar in water is physical change as the solid sugar is converted to a liquid state in water so it changes its physical state.
Classify each of the following as a physical or a chemical property. And water H 2. Rusting of iron Cooking of food Digestion of food and Burning of a candle.
A physical change is reversible a chemical change is not. Burning is a type of physical change that changes the color and odor of objects using fire and heat. No new chemical species forms in a physical change.
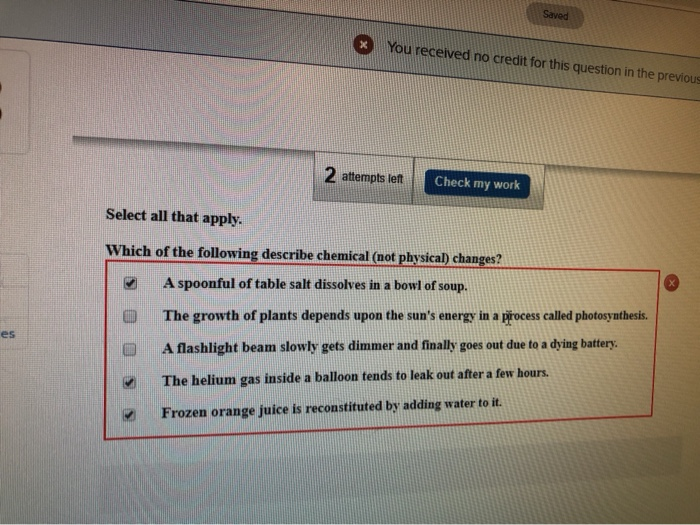
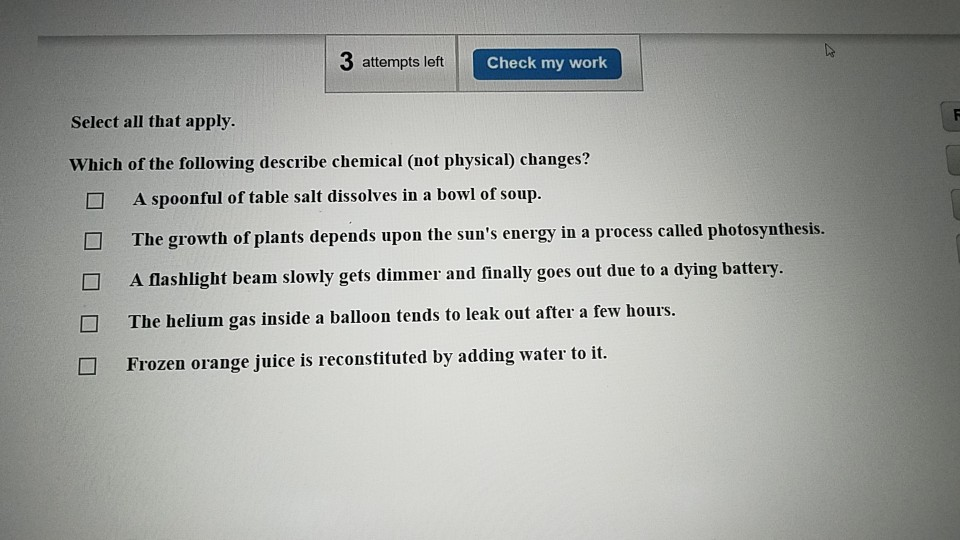
Which of the following describe chemical not physical changes. In a physical change the makeup of matter is changed. Methane burns turns into carbon dioxide water c.
Chemistry questions and answers. A flashlight beam slowly gets dimmer and finally goes out due to a. Chemical changes release energy but physical changes absorb energy.
Physical changes produce no new substances and chemical changes do. A flashlight beam slowly gets dimmer and. A physical change is a process in which the physical state of the substance is changed.
Therefore the combustion of LPG is a chemical change. Which describes how chemical changes are different from physical changes. Physical changes always involve the formation of a gas while chemical changes may not.
Since no new substances are formed and there is no loss or gain of energy it is a physical change. These are all signs of a chemical change not a physical change. If the answer is yes its a physical change.
During a chemical reaction absorption and evolution of energy take place. Chemical property changes the identity of matter while physical properties does not change the identity of matter what is the difference between accuracy and precision. Is the chemical identity of the end product the same as it was before the change.
The growth of plants depends upon the suns energy in a process called photosynthesis. A physical change involves changes in physical properties but not chemical properties. Thus chemical changes involve the formation of new substances.
Chemical because heat caused the change. Hence option C is correct. Drying of clothes is a physical change because it does not involve the creation of a new substance rather it involves removal or evaporation of water from clothes.
Iron combines with oxygen to form rust. A spoonful of table salt dissolves in a bowl of soup. Hydrogen peroxide breaks down into water and oxygen gas d.
Determine whether this change is a chemical reaction or a physical change. A spoonful of table salt dissolves in a bowl of soup. Only physical changes occurring.
Was there a color change with exceptions bubble formation or formation of a precipitate. A large change in temperature not resulting from external heating with the appearance of gas bubbles is most likely an indication of. Correct option is D LPG is flammable hydrocarbon gases that react with oxygen to form new products such as carbon dioxide CO 2.
Chemical changes can be measured but physical changes cannot. The growth of plants depends upon the suns energy in a process called photosynthesis. The growth of plants depends upon the suns energy in a process called photosynthesis.
Metallic lustre is known as a physical property because the metallic substance is not being changed. Which of the following describe chemical not physical changes. Some examples of physical change are freezing of water.
A chemical change is a permanent change. Changing the size and shapes of pieces of wood would be a chemical change. Terms in this set 40 physical salt dissolves in water chemical hydrochloric acid reacts with potassium hydroxide to produce a salt water and heat physical a piece of sodium is sliced in two physical water is heated and changed to steam chemical iron rusts chemical.
Physical changes can occur inside or outside the substance. Examples of Physical Changes. Which of the following does NOT describe a chemical change.
Changing the state of a pure substance between solid liquid or gas phase is a physical changes since the identity of the matter does not change. Remember that chemical changes form new substances physical changes do not. A physical change involves very little to no absorption of energy.
Which of the following describe chemical not physical changes. Accuracy tell how close a measurement is to the true value while precision tell how closely multiple measurement of the same thing are to one another. A chemical change is a change that brings a change in the chemical properties of matter and a new substance is formed.
Physical because no new substance was formed. Burning is a type of chemical change using fire and heat that produces new substances. For example the freezing of water would be a physical change because it can be reversed whereas the burning of wood is a chemical change you cant unburn it.
A spoonful of table salt dissolves in a bowl of soup.
Solved 3 Attempts Left Che My Work Select All That Apply Chegg Com

Solved Which Of The Following Describe Chemical Not Chegg Com

Solved Saved You Received No Credit For This Question In The Chegg Com
Comments
Post a Comment